Media – Water for Injection, Storage & Distribution
The “water for injection” produced in the MWI system or the energy saving variants MWM or MWC can be stored via the MWS system and distributed to the various consumers according to the process requirements.
Advantages and functions
- Safe and robust pure steam generation in highest quality according to pharmacopoeia for beverage, food and pharmaceutical industry
- Stainless steel in areas in contact with the product
- Feasibility studies and concept selection
- Specific boundary conditions (pharmacopoeia, TA-Luft, ATEX, GMP / FDA, DGRL, …)
- Batch operation and continuous operation
- Options
- Can be combined with MPS systems and feed water conditioning MWT
- CIP
- Manual operation up to automation
- Modular and mobile system
Technical description
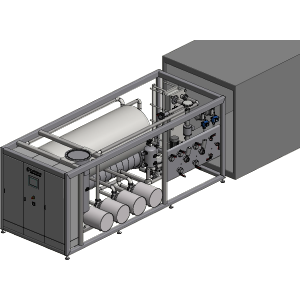 The sterile water of WFI quality is buffered in the storage tank V01 and made available to the various consumers via a ring distribution system. For this purpose, the water is pumped to the consumers by pump P01 with a sufficiently high pumping volume and inlet pressure PI. In the return line, the preservation of quality and process parameters is ensured by measuring conductivity, pressure, temperature and quantity, and optionally TOC. The temperature of the system is adjusted with the heat exchanger E01 and upstream and downstream temperature measurements. Gas exchange and waste gas is avoided as far as possible, but is generally ensured by sterile filters to prevent contamination.
The sterile water of WFI quality is buffered in the storage tank V01 and made available to the various consumers via a ring distribution system. For this purpose, the water is pumped to the consumers by pump P01 with a sufficiently high pumping volume and inlet pressure PI. In the return line, the preservation of quality and process parameters is ensured by measuring conductivity, pressure, temperature and quantity, and optionally TOC. The temperature of the system is adjusted with the heat exchanger E01 and upstream and downstream temperature measurements. Gas exchange and waste gas is avoided as far as possible, but is generally ensured by sterile filters to prevent contamination.
In addition, partial draining of the sump and CIP cleaning of the system is possible. Optional sampling according to EN285 is possible.
Technical specification
| Feed water | < 10 to 1.000 m3/h |
| Conductivity | <10 µS/cm for condensate (pharmacopoeia compliant) |
| Heating medium | process specific |
| Cooling medium | process specific |


